iStock
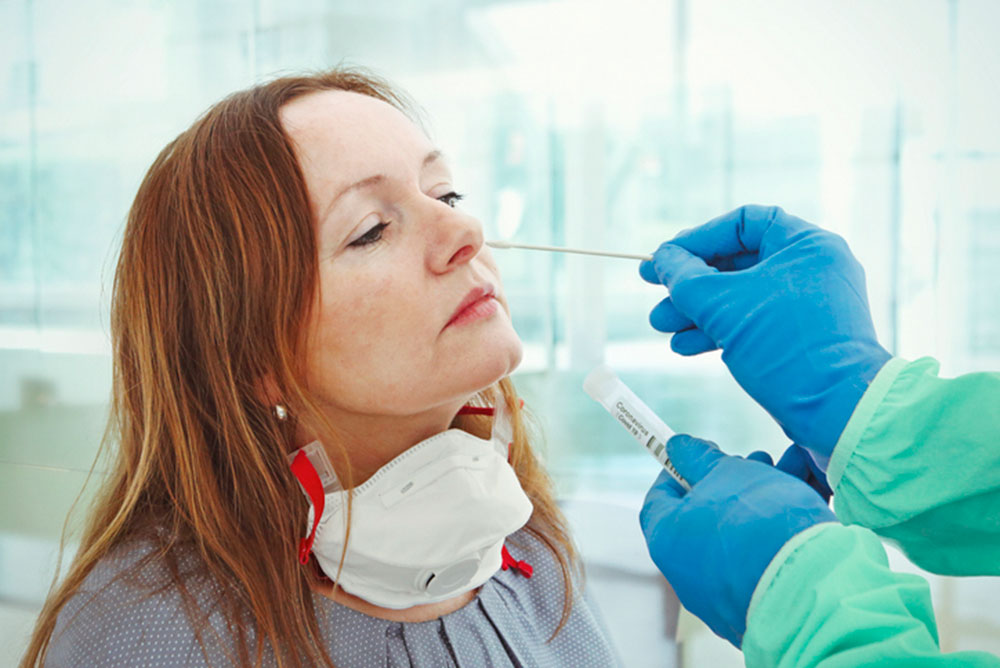
A DC–REGION childcare worker in her mid-20s, M.R. returned to work on a recent Monday with a skeleton staff to prepare for reopening before the kids arrived. On Tuesday night, she learned a colleague had just received a positive Covid-19 test result; the center had suspended reopening—after alerting everyone involved; and she needed to get tested.
For a pandemic that has been underway for more than six months, the surprisingly complicated decisions M.R.—or anyone without symptoms seeking reassurance after possible exposure–must make include how long to wait for the best chance that a test could detect the virus; where to be tested —at no cost and conveniently close to her D.C. apartment, or elsewhere; and which kind of test (among polymerase chain reaction tests for the virus, not the serological antibody test) to get.
Information at Penn Medicine—linked from a D.C. testing information site—is current but shows how murky the situation continues to be: “Since COVID-19 tests are new, knowing the accuracy is challenging. The accuracy and predictive values of SARS-CoV-2 testing have not been evaluated and the accuracy of testing depends on which test is used, the type of specimen tested, how it was collected and the duration of illness.”
Testing on the day of exposure will fail to detect active virus 100% of the time. For those who develop symptoms, a test given three days after the symptoms start will still produce negative results 20% of the time, according to Harvard Health Publishing. (Because the common swab test works by detecting genetic material from the coronavirus, positive tests are almost 100% accurate.)
Testing four days following exposure will still fail to detect active virus 40% of the time—because of the low number of viral particles in the nose or saliva by then, according to Harvard Health Publishing. And the biggest risk of false negatives is the false confidence they offer—enabling those tested, along with everyone around them, to believe they are out of the woods, not contagious and do not need to take precautions.
“What I find both frustrating and dangerous is the consistent failure to understand that testing…does little or no good,” said former CDC director Thomas Frieden. “What good is testing if the results take four days to come back and infectious people aren’t isolated in the interim?”
On contact tracing, Frieden adds, “What good is testing if contact tracing doesn’t identify and warn exposed people quickly?” The latest research suggests that “people may actually be most likely to spread the virus to others during the 48 hours before they start to experience symptoms,” according to Harvard Health.
Looking for a local test site can also be tricky, with choices varying among states and even among regions within states. For example, of four Yale-affiliated hospitals in Connecticut, three offer tests with results in seven hours while a fourth in New London takes “much longer,” according to a local health clinic.
“It may take a lab about 24 hours to run your test,” according to WebMD. But you might not get your results for several days. Future tests might be faster.” Official D.C. testing sites must mail test results rather than communicating them online or by phone.
While the CDC lists “point-of-care” swab tests that produce results on-site within an hour, there is still no evidence to support use of these tests in clinical settings, according to the Center for Evidence-based Medicine.
In D.C.,information on testing sites is in flux. Anyone with health insurance might do best to make an appointment at a medical facility rather than opting for the free fire-station test conveniently located around the corner, because most free test sites offer limited hours, the likelihood of long wait times —relevant for M.R. and others—and have strict residency requirements.
Specific CVS pharmacies offer testing but require filling out a strict questionnaire to qualify, which eliminates most people who have no symptoms or do not work in healthcare.
Alternatives to the common swab test are still in the research stage. For those that use saliva, early reports suggest fewer false negatives. In the U.S. these appear to be available only under emergency-use authorization and often with high price tags—but this situation is also in flux. Trials involving thousands of volunteers on an at-home saliva test are underway in the U.K.
In recent news, “an instant coronavirus breathalyzer,” developed by an Israeli team at Ben-Gurion University, can detect the virus in exhaled breath; produces results within a minute; and has a “success rate of 90%” in trials to date. In the handheld device, which looks like a small kazoo, a chip with densely packed sensors capture tiny particles, including viruses, in the breath.
Each device can process about 4,000 individuals a day, which allows for easy testing of travelers at airports and train stations—and reassurance seekers everywhere. The device could receive fast-track FDA approval by September.
“Strip tests” could also help with testing larger numbers of people, although positive results from these require followup from a swab or saliva test. Strip tests currently in development involve spitting into a tube and then inserting a paper strip containing proteins or another substance that detect the presence of the virus. The main advantage is cost—as low as $1 to $5 a day for regular, repeated testing—compared to $50 to $150 for each standard swab test.
For now, the U.S. is considering pooled testing—cheaper, easy to repeat regularly and most useful for schools, offices and factories. Labs take individual samples, set aside part of each sample, and combine the rest into batches of 5 or 10 samples each: if a pool yields a positive result, the lab retests the remaining part of the sample from each individual in that group.
For years, the method has worked well for screening pooled samples of donated blood, and it can increase testing capacity by at least 70%, according to a Nebraska state lab director. But pooled testing is less useful when the percent of those infected rises above 10 per 100 people, because too many samples must be retested, with an added delay of several days for each person to learn their result.
In M.R.’s case, because her exposure may have begun on the Monday morning when she started work, waiting until Friday for testing should have given her about a 60% chance of an accurate result. She also needed to begin an immediate self-quarantine—for 14 days, because the virus’s incubation period can last that long—during which, in an ideal world, to reassure herself and others, she should be able to get a new test every few days.
—Mary Carpenter
Well-Being Editor Mary Carpenter continues to update us on Covid-19. To read more of her posts, click here.

This was fascinating and full of important information I wasn’t aware of. Thank you, Mary