iStock
By Mary Carpenter
Since I last wrote about stem cell treatment for painful joints in 2017, the Arthritis Foundation has continued to warn against “unproven and likely ineffective stem cell therapies offered at the nation’s nearly 3,000 for profit clinics.” At the same time, the foundation reports that regenerative therapies, including stem cells (capable of reproducing identical or similar cells) are “the future of medicine…show promise for osteoarthritis and many other chronic diseases” —with researchers in “nearly every country…conducting clinical trials using stem cells to treat a vast array of conditions.”
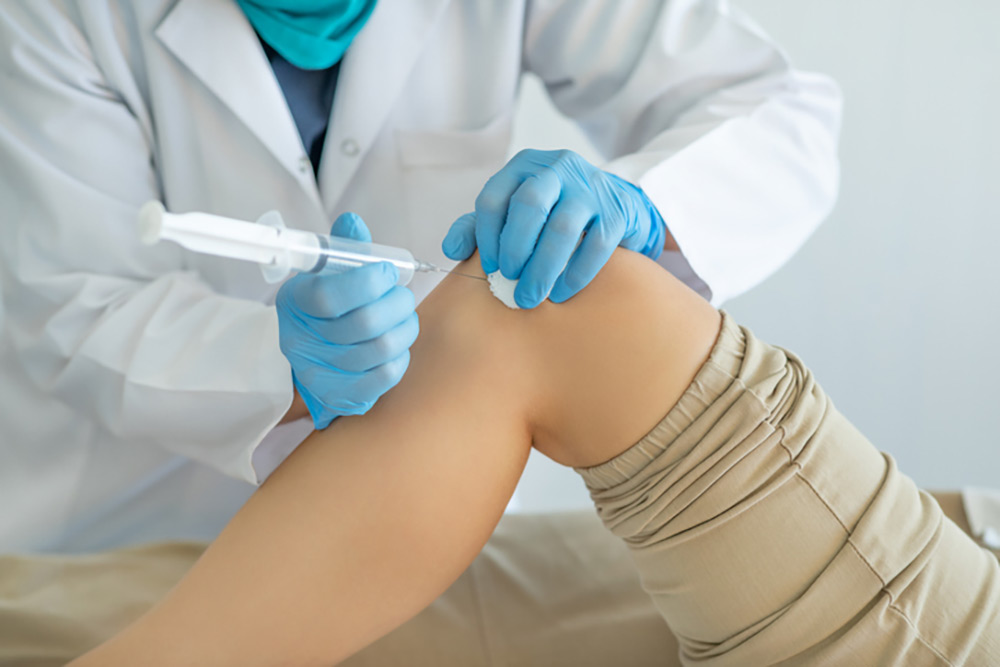
STEM CELL injections were only one of several treatments DC lawyer F.J. chose after months with a painful ankle, along with cortisone shots and physical therapy—so that when the pain finally subsided, no one knew which treatment had helped, or if they had worked in combination, or whether the passage of time played any role. And while cortisone shots can stop pain instantly, the effects wear off —with some doctors suggesting regular shots every six months or so, while others warn about risk of tissue damage.
For excruciating knee pain, Virginia horse trainer T.D. chose stem cell treatment from a Northern Kentucky chiropractor, because of the shorter recovery time compared with surgical knee replacement. Stem cells reduced her pain, sometimes completely, and she was grateful to have had that option—although the effects began wearing off after about two years. For both patients, as at most U.S. stem cell clinics, insurance did not cover the $4,000 to $7,000 cost of implantation.
“The best-case scenario I’ve heard is that stem cell injections seem to help [patients’] knees for a few months,” UCDavis stem-cell biologist Paul Knoepfler told Bloomberg. The article cites an Emory University study that concluded stem cells were no better than steroids—for which costs are usually covered by insurance—at reducing knee pain and “many studies…including a large one by the Mayo Clinic are inconclusive about whether stem cell therapy helps ailing knees.” Often, too, “what’s being billed as stem cells…can be something called amniotic extract, or dead cells.”
The FDA—which “warns consumers about unproven and likely ineffective stem cell therapies offered around the country,” according to the Arthritis Foundation—has approved them only for the treatment of certain cancers and disorders of the blood and immune system.” Meanwhile, some clarity—for the vast array of anecdotal success stories, rumors of shortages and confusion about clinical trial outcomes and oversight of quality—can come from understanding the differences among the four types of stem cells.
Most questions concerning federal regulations and stem cell shortages, for example, refer to only one category—embryonic stem cells, which exist only in the first three to five days of embryonic development and are “pluripotent,” meaning they can turn into any of the 200 cell types in the body, such as lung and brain. According to the DVC Stem organization, federal regulations restrict research to embryos created for reproductive purposes and donated by the individuals seeking reproductive treatment.
Adult stem cells, a second category, are “induced pluripotent stem cells” (iPSCs),” created from autologous cells taken from the patient’s own organs and tissues (especially bone marrow and fat) that have been “genetically reprogrammed back to an embryonic state,” according to the Arthritis Foundation. Reprogramming adult cells “would avoid ethical concerns [of embryonic cells] while guaranteeing an almost unlimited supply…but iPSCs are still in preclinical trials amid concerns about their safety and the tedious reprogramming process.”
Finally come the two best-studied kinds of stem cells—also the ones most available for treatments today. First, cord cells—human umbilical cord mesenchymal stem cells (hUC-MSCs), which are self-regenerative pluripotent cells obtained from umbilical cords removed after birth—“have the ability to alleviate pain, enhance knee joint function and potentially postpone the need for surgical intervention,” according to results from 12 clinical studies in a Chinese research survey. The cells can “promote cartilage production [and, compared with stem cells from other sources, have] “stronger proliferation, differentiation and immune regulation abilities.”
For advanced osteoarthritis, these cells “coordinate cartilage regeneration” by secreting bioactive molecules related to cartilage repair and other activities, according to a Frontiers review by other Chinese researchers. But they conclude that “there remains no clear evidence that these hUC-MSCs have a considerable advantage”—compared to the final, most common category of stem cells that come from a patient’s own bone marrow (BM-MSCs). The article cites findings that “injection of autologous BM-MSCs is safe and effective in treating knee, ankle and hip joints,” along with a more than 11-year follow-up study demonstrating “their long-term safety and efficacy…for cartilage repair.”
At Minnesota’s Mayo Clinic, the primary U.S. center for stem cell research, the RECLAIM (recycled cartilage auto/allo implantation) study uses stem cells—both autologous from the patient’s own cells, and allogenic from other donors— to promote cartilage repair for both hip and knee joints. RECLAIM, however, accepts only patients without arthritis who have had traumatic or athletic injuries—and symptomatic cartilage defects must be “fresh,” meaning very recent.
My problems with joint pain and mobility have never been serious enough for me to try regenerative treatments—at least in the absence of insurance coverage or clearer evidence that they work well. What could change my mind are the ongoing, overwhelmingly positive anecdotes from people who are pleased with the results —though, in the end, most say they cannot be entirely sure how much credit to give their stem cell treatments. But five years after his stem cell injections, DC lawyer F.J. said his discomfort is “quite minor,” a lasting effect that could not result from cortisone shots alone.
—Mary Carpenter regularly reports on topical subjects in health and medicine.
